how many electrons does lithium have|Chemistry of Lithium (Z=3) : Cebu Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe temperature at which the . Rajasthan Board of Secondary Education, Ajmer : RL/RWH/Revised Results : 2022 : Senior Secondary - 2022 Result (Last updated : 28th December 2022) Secondary & Vocational - 2022 Result . Result : 2022 : Senior Secondary(Science) - 2022 Result (Announced on 01st June 2022 at 2:00 PM)
PH0 · Lithium (Li) [3] — Chemical Element — Periodic Table
PH1 · Lithium (Li)
PH2 · Lithium
PH3 · Chemistry of Lithium (Z=3)
PH4 · 3.4: Lithium, The First Metal
Delegar en el /la Viceministra de Gestión Pedagógica del Ministerio de Educación, durante el Año Fiscal 2024, las siguientes facultades y atribuciones. Resolución Ministerial N.° 004-2024-MINEDU - Normas y documentos legales - Ministerio de Educación - Plataforma del Estado Peruano
how many electrons does lithium have*******Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe temperature at which the .
Members of a group typically have similar properties and electron configurations in .
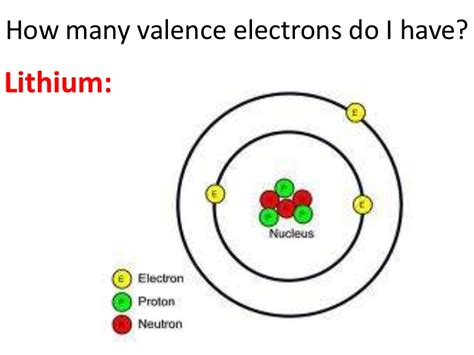
Members of a group typically have similar properties and electron configurations in .Lithium has 3 protons and electrons in its structure. A lithium-7 atom contains three protons, four neutrons, and three electrons. Lithium - Protons - Neutrons - Electrons - .The alkali metals are also called the lithium family, after its leading element. Like the other alkali metals (which are sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and .Electron affinity of Lithium is 59.6 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a .Lithium has three protons and four neutrons in its nucleus, and three electrons in two shells. It is located in group one, period two and block s of the periodic table. Socket .
Given that 7 Li is 7.0160 amu and 6 Li is 6.0151 amu, and their percent abundance is 92.58% and 7.42% respectively, what is the atomic mass of lithium? Solutions Group 17 .Lithium is a soft, white, and lustrous metal with the atomic number 3 and the electron configuration 2-1 or 1 s2 2 s1. It belongs to the alkali metal group and has many .

element. Physical data. Electronic data. Shells: 2, 1 Orbitals: [He] 2s 1Electronegativity: 1.0, 1.0 1. Ionization potential: 5.3917 eV 2. Ionization potential: 76.638 eV 3. Ionization .Learn about lithium element, its electrons, atomic number, symbol, and position in the periodic table. Find out why lithium is a metal, why it floats on water, and how it reacts .Environmental Chemistry. Green Chemistry and the Ten Commandments of Sustainability (Manahan) 3: The Elements - Basic Building Blocks of Green Chemicals. 3.4: Lithium, .
4th shell can hold 32 electrons. The atomic number of Lithium (Li) is 3. Hence, Lithium element has the electrons arrangement 2, 1. This electron arrangement indicates that the outermost orbit of .
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Therefore the Li electron configuration will be 1s 2 2s 1.
How many electrons does lithium-6 have? Updated: 8/10/2023. Wiki User. ∙ 14y ago. Study now. . How many electrons does the element Be have in it? 4 electrons, 2 valence electrons.The atomic number of lithium is 3. That is, the number of electrons in lithium is 3. Therefore, a lithium atom will have two electrons in the first shell and one in the 2nd shell. Therefore, the order of the number of electrons in each shell of the lithium(Li) atom is 2, 1. Electrons can be arranged correctly through orbits from elements 1 to 18.
Step-2: Need to do electron configuration of lithium. Step 2 is very important. In this step, the electrons of lithium have to be arranged. We know that lithium atoms have a total of three electrons. The electron configuration of lithium shows that there are two electrons in the K shell and one in the L shell.
For instance, lithium (Li ) has three electrons: two fill the 1 s orbital, and the third is placed in the 2 s orbital, giving an electron configuration of 1 s 2 2 s 1 . Neon ( Ne ), on the other hand, has a total of ten electrons: two are in its innermost 1 s orbital and eight fill the second shell—two each in the .The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital ( Figure 6.26 or Figure 6.27 ).how many electrons does lithium have In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Lithium (LI). . and neutrons for the element Lithium . Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. . the group 1 elements, including .
Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell. Therefore the lithium (Li), which has three total electrons, will have two electrons in the first shell and one electron in the second shell. Notice that lithium is the first element in the second row of the periodic table. Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5). Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons. Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons . So that means that sodium has one valence electron. And that's very convenient, because sodium is found in group one. And so we can say that for main groups, if you want to figure out .Chemistry of Lithium (Z=3) Lithium - Atomic Number. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure. . (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. 24.305 . Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li + cation. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. Lithium carbonate (Li 2 CO 3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water .
No headers. The element with atomic number 3 is lithium (Li), atomic mass 6.941. The most abundant lithium isotope is having 4 neutrons in its nucleus. A few percent of helium atoms are the isotope, which has only 3 neutrons. The third electron in lithium cannot fit in the lowest energy shell, which, as noted above, is full with only 2 electrons.
In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Lithium ion (Li+). From the Periodic Ta.
Get the latest offers and promotions from Paddy Power™! Great offers for new & existing customers can be found here: Sports Offers Casino Offers Games Offers Bingo Offers »
how many electrons does lithium have|Chemistry of Lithium (Z=3)